Since we are working with gases, the liters can be taken as moles.
According to the given balanced reaction, 1 liter of propane reacts completely with 5 liters of oxygen, use this ratio to find the liters needed to react with 37 liters of oxygen:
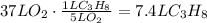
It means that 7.4 liters of propane will be needed.