There are actually no disproportionation reaction in the question. They are all incomplete and unbalanced.
Example of a disproportionate reaction:
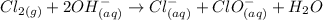
For this reaction the chlorine atom on the reactant side as an oxidation state of 0. It gets reduced to -1 in Cl- and oxidized to +1 in ClO3-