Answer:
6.67L
Explanations:
According to Boyle's law, the pressure of a given mass is inversely proportional to its volume provided that the temperature remains constant. Mathematically;
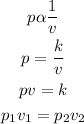
where:
• p₁ and p₂ are the, initial and fina,l pressure
,
• v₁ and v₂ are the ,initial and final volume
Given the following parameters
p₁ = 20atm
p₂ = 30atm
v₁ = 10L
Required
new volume v₂
Substitute the given parameters into the formula to have;
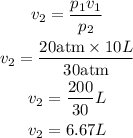
Therefore the new volume of the gas is 6.67L