Step-by-step explanation
Volume of tank with water = 1 kL
Volume of tank (water + Mr. C) = 1.25 kL
Volume Mr. C = 1.25 kL - 1 kL = 0.25 kL
It is known that 1 kL = 1000 L and 1 L = 1000 mL
So,
0.25 kL x (1000 L/1 kL) x (1000 mL/1 L) = 250000 mL
-----
Now, it is also known that density can be calculated as follows:
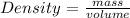
Therefore,
Density = Mass of Mr. C/Volume of Mr. C
(First, 1 kg = 1000 g, so 100 kg x (1000 g/1 kg) = 100000 g)
----
Density = 100000 g/250000 mL = 0.4 g/mL
Answer: 0.4 g/mL