ANSWER
The number of moles of K3P is 0.444 moles (OPTION A)
Explanation:
Given information
The mass of K3P is 65.8 grams
Let the number of moles be m
To find the number of moles, we will need to apply the below formula
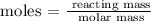
The next step is to find the molar mass of K3P
According to the periodic table, the molar mass of potassium is 39.0983 g/mol, and the molar mass of phosphorus is 30.97 g/mol
Since we have gotten the molar mass of each element, then, it is easy to find the molar mass of the compound.

Since we have gotten the molar mass of the compound, then, we can now find the number of moles
