ANSWER
Acidic
Step-by-step explanation
Ammonium chloride is an acidic salt prepared the combination of a strong acid and a weak base
The strong acid is the hydrochloric acid and the weak base is the ammonium hydroxide.
Below is the reaction between hydrochloric acid and ammonium hydroxide
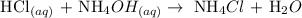
Therefore, the pH of NH4Cl in solution is acidic.