Step 1
Molarity is defined as follows:
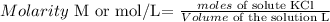
L means litters.
----------------------
Step 2
Information provided:
0.345 moles KCl
345 mL of solution x (1 L/1000 mL) = 0.345 L
(1 L = 1000 mL)
-----------------------
Step 3
Then, molarity: from step 1
Molarity = 0.345 moles/0.345 L = 1 M or 1 mol/L
Answer: Molarity = 1 M