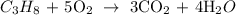Answer:
Step-by-step explanation:
Here, we want to get the balanced equation for the combustion of propane
When propane is burned in an unlimited supply of oxygen, what we have produced is carbon (iv) oxide and water
We have the complete and balanced chemical equation as follows: