1) List the known and unknown quantities.
Sample: Glucose.
Density: 1.15 g/mL.
Volume: 2.31 gal.
Mass: unknown (kg).
2) Convert mL to gal.
1 gal = 3785.41 mL.
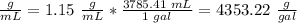
3) Find the mass
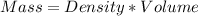
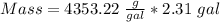
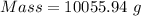
4) Convert g to kg.
1000 g = 1 kg.
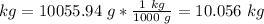
The mass of 2.31 gal of glucose solution is 10.06 kg.