Given:
The mass of ice is,
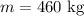
The initial temperature of ice is,
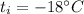
The heat for this ice to become 0 degree C ice is,
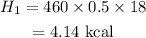
The heat needed for 0 degree ice to 0 degree water is,
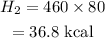
Now the heat for the final step to reach 20 degrees is,
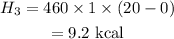
The total heat is,
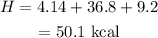
Hence the total heat is 50.1 kcal.