Answer
d. 63.95 g
Step-by-step explanation
Given:
Mass of potassium chlorate = 163.27 g
Equation: 2KCIO₃ → 2KCl +3O₂
What to find:
The mass of oxygen that can be produced from heating 163.27 g of KCIO₃.
Step-by-step solution:
From the given equation, 2 mol KClO₃ produced 3 mol O₂
Molar mass of KCIO₃ = 122.55 g/mol
2 mol KClO₃ = 2 mol x 122.55 g/mol = 245.10 g
Molar mass of O₂ = 31.998 g/mol
3 mol O₂ = 3 mol x 31.998 g/mol = 95.994 g
Therefore the mass of oxygen produced is calculated as follows:
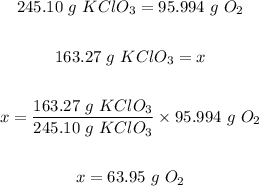
Thus, the mass of oxygen obtained from heating 163.27 g of KCIO₃ is 63.95 g.
The correct answer is option d. 63.95