The question requires us to calculate the concentration of a NaOH solution, given the amount and concentration of HCl required to neutralize 50.0 mL of this base solution.
The following information was provided by the question:
concentration of HCl solution = C(HCl) = 0.950 M = 0.950 mol/L
volume of HCl solution = V(HCl) = 80.0 mL
volume of NaOH solution = V(NaOH) = 50.0 mL
To solve this problem, we need to understand what happens when NaOH and HCl react. A neutralization reaction occurs between a strong acid, such as HCl, and a strong base, such as NaOH, where the amount of H+ and OH- ions in solution are equal. We can write their reaction as:
NaOH + HCl -> NaCl + H2O
When the base is completely neutralized by the acid, it means that:
number of moles of NaOH = number of moles of HCl
The equality above is what we'll use to start our calculations.
Another important information to this question is that, by definition, the molar concentration is the number of moles of a compound divided by the volume of the solution:
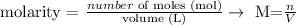
Since we have the equality between the number of moles of acid and base, we can rewrite the equation above as:
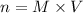
and use this to calculate the molar concentration (M) of NaOH.
Thus, so far we have that:
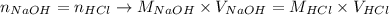
Since the volume of NaOH, molarity of HCl and volume of HCl were provided by the question, we can rearrange the equation above to calculate the molarity of NaOH:
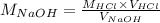
And, at last, we can apply the values provided by the problem (note that the volume here is being used in mL instead of L; this is fine as long as the volume of both solutions, acid and base, are used with the same unit):
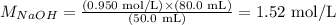
Therefore, the molar concentration of the NaOH solution is equal to 1.52 mol/L (or 1.52 M).