ANSWER
The mass of water produced during the reaction is 2.0 grams
Step-by-step explanation
Given that;
The mass of octane reacted is 5.71 grams
The mass of oxygen reacted is 5.0 grams
Follow the steps below to find the mass of water produced during the reaction
Step 1; Write a balanced equation of the reaction
Recall, that the major products formed when organic compounds undergo combustion reaction are water and carbondioxide
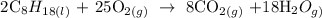
Step 2; Find the number of moles of octane and oxygen using the below formula
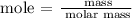
Recall, that the molar mass of octane is 114.23 g/mol and the molar mass of oxygen is 32 g/mol
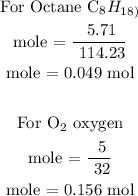
In the above calculations, the mole of octane is 0.049 mole and the number of mole of oxygen is 0.156 mol
Step 3; Find the limiting reactant of the reaction
To determine the limiting reactant of the reaction, divide the number of mole of the reactant by its coefficient.
Recall, that the coefficient of octane is 2 and the coefficient of oxygen is 25
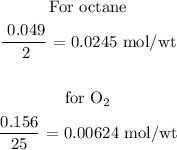
The limiting reactant is oxygen because it has the least mol/wt
Step 4; Find the number of moles of water using a stoichiometry ratio
25 moles O2 react to give 18 moles H2O
Let x represent the number of moles of H2O
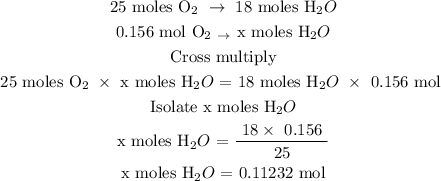
The number of moles of water is 0.11232 mol
Step 5; Find the mass of water using the below formula
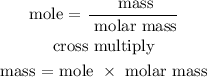
Recall, that the molar mass of water is 18 g/mol
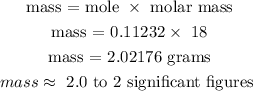
Therefore, the mass of water produced during the reaction is 2.0 grams