This is a combined gas law problem. Let's see the formula of combined gas law:
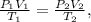
where P is pressure, V is volume and T is temperature. In this formula, the units of each variable must be: for pressure, atm, for volume, in liters (L), and for temperature, in kelvin (K).
Remember that 1 kPa is 0.0986 atm, and kelvin is calculated summing 273 for celsius.
Let's calculate pressure:
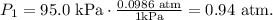
And the formula for temperature in kelvin is:
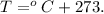
For our initial temperature, we have:
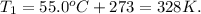
So, the initial data will be:
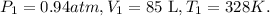
Now, let's do the conversions for the final data. For pressure, we have:
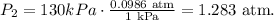
So, our final data is:
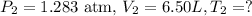
The problem is asking for the temperature in Celsius.
The first step is clear temperature (2) of the formula of combined gas law that is at the beginning:
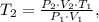
Then, you have to replace the given data, like this:
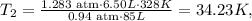
But remember that this answer is in kelvin, so now, we need to clear the formula of temperature to obtain the result in Celsius. The formula is:
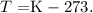
And replacing the result, we can calculate the temperature in Celsius, like this:
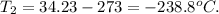
So, the answer will be -238.8 C, because when volume decreases, the temperature decreases (volume and temperature are directly proportional), and when pressure is increasing, the temperature decreases (pressure and temperature are inversely proportional).