Answer
0.509 grams Al³⁺
Step-by-step explanation
Given:
Mass of aluminum cyanide = 1.98 grams
Required: To find the grams of Al³⁺ present in 1.98 grams of aluminum cyanide.
Step-by-step solution:
The grams of 1 mole of aluminum cyanide (Al(CN)₃) = 105.03 grams
This implies that 27.0 g Al³⁺ is present in 105.0 grams of Al(CN)₃
So, the grams of Al³⁺ present in 1.98 grams of Al(CN)₃ will be:
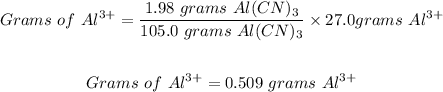
Thus, 0.509 grams of Al³⁺ are present in 1.98 grams of aluminum cyanide.