Answer:
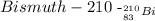
Explanations:
If the atom of Astatine-214 undergo alpha particle, it will emit helium element as shown;
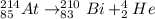
According to the decay, 4 is subtracted from the mass number and 2 from the atomic number.
According to the reaction, the new isotope produced is Bismuth-210