Answer:
The work is -95445 J.
Step-by-step explanation:
First, let's see the formula of work:
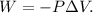
Where W is work, P is pressure, and ΔV is the change of volume (ΔV = Final volume - Initial volume).
As we have the given data, we can replace what we have in the formula, like this:
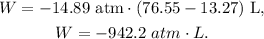
But remember that the answer must be in joules (J). Remember that 1 atm-L equals 101.3 J:
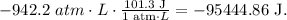
The answer would be that the work is -95445 J.