Answer
Therefore, the mass in grams of NaCl in the solution = 0.1 grams
Step-by-step explanation
Given:
Volume = 25 mL
Concentration of the solution in mg/mL = 4 mg/mL
What to find:
The mass in grams of NaCl in the solution.
Solution:
Concentration, C in mg/mL = Mass, (m) / Volume (V) i.e
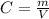
Putting C = 4mg/mL, and V = 25 mL into the formula, we have
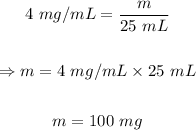
The final step is to convert 100 mg to grams.
Conversion factor:
1 mg = 10⁻³ grams
So 100 mg ⇒ (100mg/1 mg) x 10⁻³ grams = 0.1 grams.
Therefore, the mass in grams of NaCl to make the solution = 0.1 grams.