Step 1 - Discover which reactants or products are part of the equilibrium constant
The given reaction is the dissolution of Li2CO3, which can be represented as:
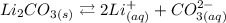
In an equilibrium constant, only liquid, aquous or gas reactans or products will be considered. Therefore, Li2CO3, being a solid, will not be part of the dissolution equilibrium constant.
Step 2 - Set the equilibrium constant for the reaction
We know that an equilibrium constant is given by the quocient between products and reactants. In this case, since our reactant is a solid, it will not be taken into account. We have, then:
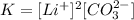
Remember: the concentration of Li(+) must be elevated to the power of 2 because, in the dissolution reaction, two moles of Li(+) are formed.