Answer:
Explanation
GIVEN
• Mass of Al2O3 =, 408g
• Molecular mass Al2O3 =,101,96 g/mol
• Molecular Mass Oxygen =,15,999 g/mol
We will consider the following balanced chemical equation that takes place :
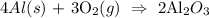
( i) Calculate the moles of Al2O3
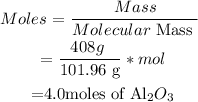
(ii) Determine moles of Oxygen from the stoichiometry in the balanced reaaction
3 moles Oxygen reacts and produce 2 moles Al2O3
So, x moles Oxygen willreact and produce 4 moles Al2O3
Therefore,
X moles O2 = (4Moles Al2O3 *3 moles Oxygen ) / 2 moles Oxygen
= 6 moles of O2
(iii) Determine Mass of Oxygen :
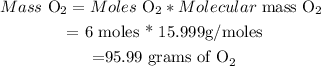
Therefore, 95.99 grams of O2 are needed to produce 408g of Al2O3