ANSWER
The molarity of NaOH is 0.933 M
Step-by-step explanation
Given that;
The volume of NaOH is 30.00mL
The volume of H2SO4 is 35.00mL
The molarity of H2SO4 is 0.400 M
The number of moles of the acid (nA) = 1
The number of moles of the base(nB) = 2
The balanced equation of the reaction is
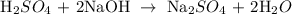
Follow the steps below to find the molarity of NaOH
Step 1; Apply the dilution formula
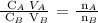
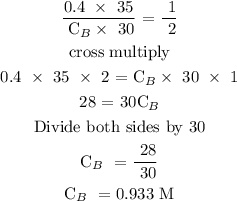
Therefore, the molarity of NaOH is 0.933 M