Given:
The initial volume of the balloon, V₁=457 cm³=457×10⁻⁶ m³
The initial temperature of the balloon, T₁=270 K
The final temperature of the balloon, T₂=585 K
To find:
The new volume of the balloon.
Step-by-step explanation:
From Charle's law,
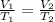
Where V₂ is the new volume of the balloon.
On substituting the known values,
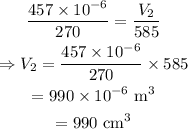
Final answer:
Thus the new volume of the balloon is 990 cm³