Answer:
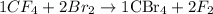
Explanations:
Given the unbalanced chemical reaction:
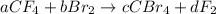
You need to determine the values of the constants a, b, c, and d that will balance the chemical reaction.
Note that to balance the reaction, the number of moles of elements on both sides of the reaction must be equal.
For Carbon elements:
a = c
For Flourine element:
4a = 2d
2a = d
For Bromine element:
2b = 4c
b = 2c
Recall that a = c, therefore, b = 2a
Replace c = a, b = 2a and d = 2a into the reaction to have:
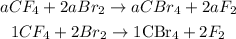
This gives the balanced equation for the chemical reaction.