We are given the balanced formula for the reaction so we can continue with the calculations.
A. To calculate the mass of AlBr3 formed from 5.6 g of Al we will do the following calculations:
1. We find the moles corresponding to 5.6 grams of Al. To do this, we divide the 5.6 grams by the molar mass of aluminum. The molar mass of Al is 26.98g/mol.
2. We find the moles of AlBr3 produced. From stoichiometry we know that two moles of Al produce two moles of AlBr3, so the AlBr3/Al ratio is 2/2.
3. We find the grams of AlBr3 by multiplying the moles of AlBr3 found by the molar mass. The molar mass of AlBr3 is:266.69g/mol.
So, the grams of AlBr3 formed from 5.6 g of Al will be:
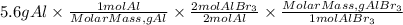
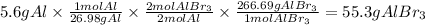
Answer A. the mass of aluminum bromide that can be produced from 5.6 g of Al is 55.3 grams.
B. To calculate the mass of AlBr3 produced from 4.4 grams of Br2 we follow steps 1, 2, and 3 described above.
In step 1, we will use the molar mass of Br. The molar mass of Br2 is 159.808g/mol.
In step 2, we will taka in count that the ratio AlBr3 to Br2 is 2/3
So, the mass of AlBr3 will be:
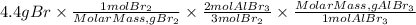
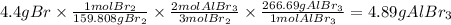
Answer B. the mass of aluminum bromide that can be produced from 4.4 g of Br is 4.9 grams.
C. The percent yield will be found with the following reaction:
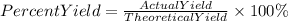
We will use the minor value of AlBr3 found. It means we will use as theoretical yield 4.9 grams of AlBr3.
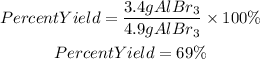
Answer C. The percent yield is 69%
In summary, we will have:
A. The mass of aluminum bromide that can be produced from 5.6 g of Al is 55.3 grams.
B. The mass of aluminum bromide that can be produced from 4.4 g of Br is 4.9 grams.
C. The percent yield is 69%