The enthalpy change is the difference in the energy of formation of the products minus that of the reactants, we have the following formula:
ΔHr = Σn*ΔH (products) - Σn*ΔH (reactants)
Now, we are given the values for the energy of formation of all the compounds that participate in the reaction. We must always make sure that the reaction equation they give us is balanced, in this case, it is balanced since the atoms of each element are the same on each side of the reaction.
Now, let's calculate the energy of the formation of the products and reactants separately.
For the products we will have to:
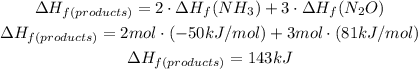
For the reactants we will have to:
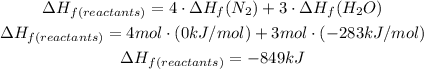
So, the change of enthalpy will be:
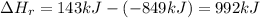
Enthalpy change is 992kJ