Answer:
1.5g of H2 are required.
Step-by-step explanation:
1st) From the balanced reaction we know that 3 moles of H2 react with 1 mole of Fe2O3.
Using the molar mass of H2 (2g/mol) and Fe2O3 (160g/mol), we can calculate the grams of H2 that are needed to react with 80g of Fe203.
- 1 mole of Fe2O3 weighs 160g.
- 3 moles of H2 weight 3g.
2nd) With the grams and a mathematical rule of three we can calculate the amount of H2 needed:
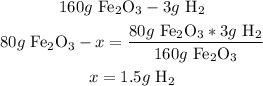
So, 1.5g of H2 are required.