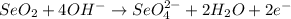Answer
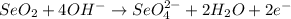
Step-by-step explanation
The given balanced half-reaction for an acidic solution:
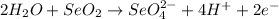
What to find:
Tha balanced half-reaction for a basic solution.
Step-by-step-solution:
To balance the half-reaction for a basic solution;
1. Add OH⁻ ions to BOTH SIDES to neutralize any H⁺
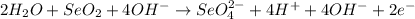
2. Combine H+ and OH- to make H2O.
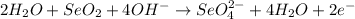
3. Simplify by canceling out excess H2O
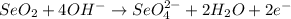
4. Balance the charges by adding e-