Step 1 - Remembering the molar mass of glycine and relating it to the number of molecules
Remember the molar mass of glycine is 75.05 g/mol. In our previous session, we have discussed its meaning: one mole of glycine weights 75.05 g.
One mole also represents a specific quantity: 6.02*10^23 unities of something. Since we're talking glycine (a molecule), one mole of glycine represents 6.02*10^23 molecules of glycine.
Therefore, we can say that 6.02*10^23 molecules of glycine (1 mole) weights 75.05 g.
Step 2 - Finding the number of molecules
Since we know the weight of 6.02*10^23 glycine molecules, we can now set the following proportion:
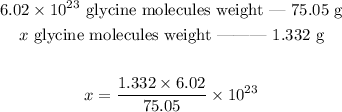
Working out the math:
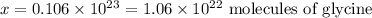
There are thus 1.06*10^22 molecules of glycine in 1.332 g of it.