1) Balance the chemical equatio.
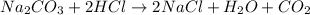
2) Which is the limiting reactant.
2.1- How many moles of HCl d we need to use all of the Na2CO3?
The molar ratio between HCl and Na2CO3 is 2 mol HCl: 1 mol Na2CO3.
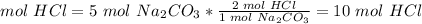
We need 10 mol HCl and we have 8 mol HCl. We do not have enough HCl. This is the limiting reactant.
2.2- How many moles of Na2CO3 do we need to use all of the HCl?
The molar ratio between HCl and Na2CO3 is 2 mol HCl: 1 mol Na2CO3.
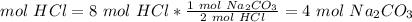
We need 4 mol Na2CO3 and we have 5 mol Na2CO3. e have ebnough Na2CO3. This is the excess reactant.
3) Moles of NaCl produced.
Limiting reactant: 8 mol HCl.
The molar ratio between HCl and NaCl is 2 mol HCl: 2 mol NaCl.
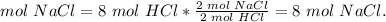
4) Grams of NaCl produced.
The molar mass of NaCl is 58.44 g/mol.
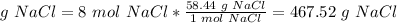
d. 467.52 g NaCl can be produced.
5) Grams of excess reactant react and get used.
In step 2.2 we got the result below
We need 4 mol Na2CO3 and we have 5 mol Na2CO3. We have enough Na2CO3. This is the excess reactant.
According to this resu4t, mol Na2CO3 reacts and gets used up and 1 mol remains in excess.
5.1- Convert moles to grams (reacted)
4 mol Na2CO3.
The molar mass of Na2CO3 is 105.99 g/mol
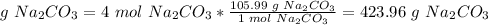
e. 423.96 g react and get used up.
5.2-Convert moles to grams (excess)
1 mol Na2CO3.
The molar mass of Na2CO3 is 105.99 g/mol
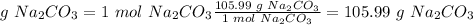
f. 105.99 g of the excess reactant remains in excess.