Answer:
a) Equilibrium shift to the left
b) Equilibrium shift to the left
c) Equilibrium shift to the left
Explanations:
Given the chemical reaction shown below;
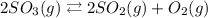
The reactant side is SO3 while the product side are SO2 and O2.
• Increasing the concentration of the reactant and decreasing the concentration of the product will shift the equilibrium position to the right.
• Decreasing the concentration of the reactant and, increasing the concentration of the product ,will, shift the equilibrium position to the left.
Based on the above conclusion, adding sulphur dioxide to the system (increasing concentration of product) will shift the equilibrium position to the left.
Removing sulphur trioxide means reducing the concentration of the reactant. This will shift the equilibrium position to the left since there will be low concentration of gas at the reactant.
Also, adding oxygen to the system (increasing concentration of product) will shift the equilibrium position to the left.