The first step is to find the concentration of acetate in the solution, to do it, convert the given mass to moles using sodium acetate molar mass:
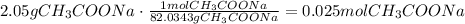
Now, divide the amount of moles by the volume in liters:
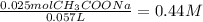
Finally use the equation of Henderson-Hasselbach to calculate the pH
