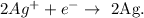We have the chemical equation:
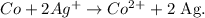
You can realize that the cobalt (Co) in the reactant doesn't have any charge, but in the product is losing two electrons (it is a cation), this means that is oxidating. The oxidation half-reaction is:
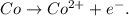
And the Ag (silver), is gaining one electron, because in the reactants loses one electron per 2 moles of Ag. The reduction half-reaction is: