Answer:
Approximately
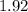 .
.
Step-by-step explanation:
The
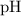 of a solution is the opposite of the base-
of a solution is the opposite of the base-
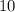 logarithm of that solution's
logarithm of that solution's
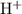 concentration measured in
concentration measured in
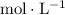 . In other words:
. In other words:
![{\rm pH} = - \log_(10) ([{\rm H^(+)])](https://img.qammunity.org/2023/formulas/chemistry/high-school/nd0miqzcrdkb8xk9ciqyn8qlrb392iwm06.png) .
.
Using properties of logarithms:
![\begin{aligned}{\rm pH} &= - \log_(10) ([{\rm H^(+)]) \\ &= -\left(\frac{\ln([{\rm H^(+)]})}{\ln(10)}\right)\end{aligned}](https://img.qammunity.org/2023/formulas/chemistry/high-school/io0h7fhhdohhxq01696ekwkww43srr97pk.png) .
.
In this question,
![[{\rm H^(+)}] = 1.2 * 10^(-2)\; {\rm mol \cdot L^(-1)}](https://img.qammunity.org/2023/formulas/chemistry/high-school/bxvx0xhrozpjbxdj08ksn2zxn9tw5sa92d.png) . Therefore:
. Therefore:
![\begin{aligned}{\rm pH} &= - \log_(10) ([{\rm H^(+)]) \\ &= -\left(\frac{\ln([{\rm H^(+)]})}{\ln(10)}\right) \\ &= - (\ln(1.2 * 10^(-2)))/(\ln(10)) \\ &\approx 1.92\end{aligned}](https://img.qammunity.org/2023/formulas/chemistry/high-school/bluxvh34paua4lihko8b47vo74h3opjkd4.png) .
.
By convention, the number of decimal values in the
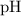 should be equal to the number of significant figures in the
should be equal to the number of significant figures in the
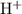 concentration of the solution.
concentration of the solution.
In this question, there are two significant figures in the measurement
![[{\rm H^(+)}] = 1.2 * 10^(-2)\; {\rm mol \cdot L^(-1)}](https://img.qammunity.org/2023/formulas/chemistry/high-school/bxvx0xhrozpjbxdj08ksn2zxn9tw5sa92d.png) . Thus, the
. Thus, the
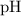 result should be rounded to two decimal places.
result should be rounded to two decimal places.