We are required to calculate the percentage yield of sodium carbonate.
We are given this reaction:
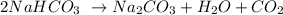
Given: mass of NaHCO3 = 1.357 g and molar mass =84.01g/mol
mass of Na2CO3 = 0.768g and molar mass = 105.99 g/mol
%Yield = (actual yield/theoretical yield) x 100
We do know the actual yield, which is 0.768 g, but we do not know the theoretical yield.
number of moles of NaHCO3 = 1.357 g/84.01 g/mol
=0.01615 mol
Take the number of moles and multiply by molar ratio and multiply by molar mass of Na2CO3 to the theoretical mass of Na2CO3.
mass of Na2CO3 = 0.01615 mol x (1/2) x 105.99 g/mol
= 0.856 g
%Yield = (0.768/0.856) x 100
%Yield = 89.7%
.