This process can be modeled by the next formula:
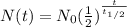
where N(t) is the quantity of the substance remaining, N0 is the initial quantity of the substance, t is the time elapsed, and t1/2 is the half-life of the substance.
Substituting with N0 = 960 grams, t = 500 days, and t1/2 = 250 days, we get:
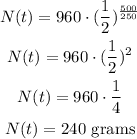
There will be 240 grams