Answer
295.31 grams of O₂ are needed to completely burn 81.4 g C₃H₈.
Step-by-step explanation
Chemical equation of the combustion reaction
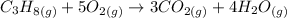
Molar mass of oxygen = 15.999 g/mol
Molar mass of propane = 44.1 g/mol
From the balanced reaction above, 1 mole propane reacts with 5 moles oxygen to produce 3 and 4 moles of carbon dioxide and water respectively.
⇒ (5 x 2 x 15.999) g = 159.99 g O₂ completely burn 44.1 g C₃H₈,
Therefore, the grams of O₂ needed to completely burn 81.4 g C₃H₈, will be
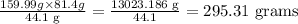
295.31 grams of O₂ are needed to completely burn 81.4 g C₃H₈.