INFORMATION:
We have 34.2g of sodium carbonate, and we must convert to molecules
STEP BY STEP EXPLANATION:
To convert to molecules, we need to:
1. Calculate the number of moles in the 34.2 g of sodium carbonate, using that the molar mass of sodium carbonate is 105.9888 g/mol
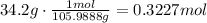
2. Using the moles, we can calculate molecules multiplying by 6.023x10^23/mol
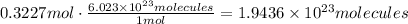
Finally, there are 1.9436x10^23
ANSWER:
34.2g of sodium carbonate = 1.9436 x 10^23