ANSWER
The pressure of the gas is 2.82 atm
Step-by-step explanation
Given that;
The moles of the gas is 0.30 moles
The volume of the gas is 2.53L
The temperature of the gas is 290K
Follow the steps below to find the pressure of the gas
Assume is the gas behaves like an ideal gas
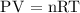
Substitute the given data into the formula above

Therefore, the pressure of the gas is 2.82 atm