We will assume that the gas behaves like an ideal gas, so we can apply the following equation for ideal gases:

Where,
P is the pressure of the gas in atm, 7.7394 atm
V is the volume of the gas in liters, 26.22L
R is a constant, 0.08206atm.L/mol.K
T is the temperature of the gas in Kelvin, 423°C=696.15K
n is the number of moles
Now, we clear the number of moles and replace the known data:
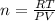
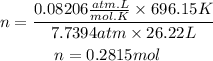
Answer: There are 0.2815 mol of carbon dioxide