Answer
0.009 mol/L
Step-by-step explanation
Given:
Mass of KCl = 0.66 g
Volume of water = 700 mL = 0.7 L
From the periodic table: 1 mol K = 39.10 g; 1 mol Cl = 35.453 g
What to find:
The molarity of the solution
Step-by-step solution:
The formula to calculate molarity is:
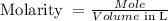
The first step is to calculate the molar mass of KCl
KCl = Mass of 1 mol K + Mass of 1 mol Cl
KCl = 39.10 g + 35.453 g
KCl = 74.553 g
So the molar mass of KCl = 74.553 g/mol
The next step is to determine the number of moles of KCl in 0.66g of KCl:
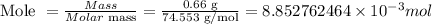
Put the values of mole and volume into the molarity formula above to determine the molarity of the solution:
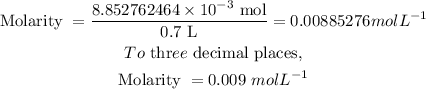
The molarity is 0.009 mol/L