Answer
The Balanced reaction is: Fe₂O₃ + 3C → 2Fe + 3CO
The grams of iron (Fe) produced = 48.96 grams.
Step-by-step explanation
Given:
Mass of Fe₂O₃ used = 70.0 grams
What to find:
The grams of iron (Fe) produced.
Step-by-step solution:
The balanced reaction between iron(III) oxide and pure carbon in a blast furnace to produce molten iron metal and carbon monoxide is:
Fe₂O₃ + 3C → 2Fe + 3CO
The next step is to convert 70.0 grams of Fe₂O₃ to moles using the mole formula
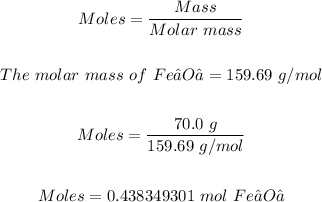
Now, we can use the moles of Fe₂O₃ and the mole ration from the equation above to determine the moles of Fe produced.
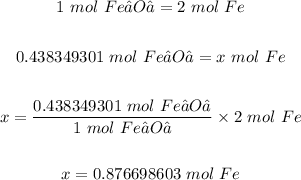
Finally, we can convert 0.876698603 mol Fe to mass in grams as follows
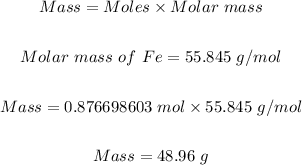
The grams of iron (Fe) produced is 48.96 grams.