The first step to solve this problem is to find the number of moles of NaCl in 50.0mL of 0.100M NaCl. To do it, convert the volume of solution to liters and multiply it by the concentration of the solution (M=mol/L).
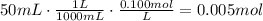
Now, use the molar mass of NaCl to find the mass of 0.005moles of it (MM=58.44g/mol):
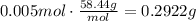
It means that you have to add 0.2922g to 50mL of water.