Step 1
When H2O is added to a solution, it gets diluted.
Mathematically:
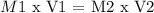
M1 = concentration 1 of the solution
V1 = volume 1 of the solution
---
M2 = new concentration of the solution
V2 = new volume of the solution
-----------------
Step 2
Information provided:
M1 = 20.8 M HCl
V1 = 60.0 mL
--
M2 = unknown
V2 = 600. mL
-----------------
Step 3
From step 1, M2 is found as follows:
M1 x V1/V2 = M2
(20.8 M x 60.0 mL)/600. mL = M2
2.08 M = M2
Answer: M2 = concentration 2 = 2.08 M