Answer:
O2 will be the limiting reactant while CS2 will be the excess reactant
Explanations:
Given the reaction between CS2 and oxygen expressed as:
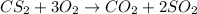
Given the following pramters
Mass of CS2 = 20grams
Mass of O2 = 10grams
Determine the mole of CS2
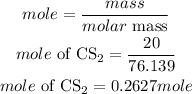
Determine the mole of oxygen
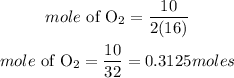
Since there are 3 atoms of oxygen, 1 atom will have:
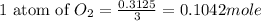
Since the mole of oxygen is less than the mole ofCS2, hence O2 will b the limiting reactant while CS2 will be the excess reactant