Answer:
a) Upon adding silver nitrate, a white precipitate is observed
b) Upon adding ammonium hydroxide, the white precipitate dissolves to give a clear, colorless solution
Step-by-step explanation:
Here, we want to state the observations when testing for chloride ions
From what we have:
a) When silver nitrate is added, a white precipitate is formed
This is as a result of the following chemical reaction:
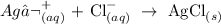
The AgCl is the white precipitate formed
b) Upon the addition of the ammonium hydroxide solution, a colorless and clear solution is observed showing that the white precipitate has dissolved