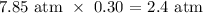Answer:
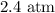
Step-by-step explanation:
Here, we want to get the partial pressure of the chlorine gas
The total mole fraction is 1
The total mole fraction is the sum of the individual mole fractions
To get the mole fraction of chlorine, we have it that:
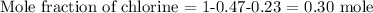
The partial pressure is the product of the mole fraction and the total pressure
Mathematically, that would be: