The reaction it describes is given by the following balanced equation:
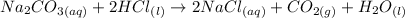
We see that two moles of HCl react to form 1 mole of CO2. They further tell us that the moles present in the HCl solution react completely, so the acid will be the limiting reagent. To find the concentration of the HCl solution we will follow the following steps:
1) We calculate the moles of CO2 from the grams that are formed, 19.1 gCO2. To do this, we divide the mass by the molar mass of CO2. The molar mass of CO2 is 44.01g/mol.
2) By stoichiometry we find the moles of HCl that must react to form the moles of CO2 found. We will take into account that the HCl to CO2 ratio is 2/1.
3)We find the molarity of the solution by dividing the moles of HCl by the volume of the solution used in liters, 601mL=0.601L.
Let's proceed with the calculation.
1. Moles of CO2
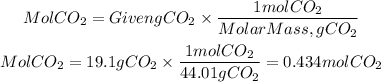
2. Moles of HCl
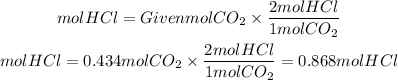
3. Concentration (Molarity) of HCl solution
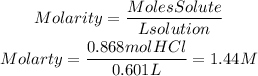
The concentration of HCl solution was 1.44 M
Answer:
Value --->1.44
Units --- > M