Answer:
There would be 5.7x10^58 grams.
Step-by-step explanation:
1st) It is necessary to convert the 3.49x10^80 molecules to moles, by using the Avogadro's number. The A vogadro's number is 6.022x10^23 particles, in this case molecules, that are contained in 1 moles of a substance:
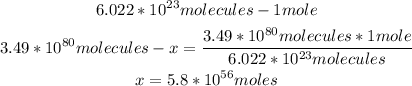
2nd) Now with the number of moles and the molar mass of H2SO4 (98g/mol) we can calculate the grams:
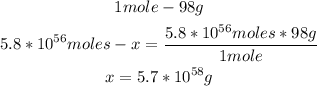
So, there would be 5.7x10^58 grams.