Answer:
14.05moles
Explanations:
According to the Avogadro constant
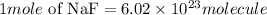
Given the following parameter
Molecules of NaF = 8.46 x 10^24molecules
Determine the moles of NaF
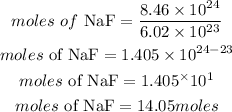
Hence the moles of NaF that are in 8.46 x 10^24 molecules of sodium fluoride is 14.05moles