Answer
0.113 M
Step-by-step explanation
Given that:
The volume of the stock solution, V₁ = 50.0 mL
The concentration of the stock solution, C₁ = 1.74 M
The volume of the solution the chemist made, V₂ = 770.0 mL
What to find:
The concentration (C₂) of the BaCl₂ solution the chemist made.
Step-by-step solution:
The concentration (C₂) of the chemist's working solution can be calculated using the dilution formula below:
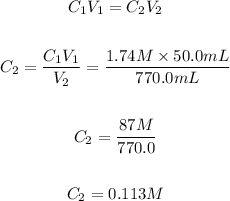
Hence, the concentration of the chemist's working solution is 0.113 M.